Background: Chronic graft versus host disease (cGVHD) is a major cause of morbidity and late non-relapse mortality after allogeneic hematopoietic cell transplantation and is commonly associated with prolonged immune suppression. Patients (pts) with inadequate response to steroids have few effective therapeutic options and represent an unmet medical need. Available therapies are associated with significant toxicity, immunosuppression, and increased risk of infections. Preclinical studies demonstrate that CSF-1/CSF-1R is a key regulatory pathway involved in the expansion and infiltration of donor-derived macrophages that mediate cGVHD. Axatilimab (SNDX-6352, axa) is a humanized, full-length IgG4 antibody with high affinity to CSF-1R. Axa affects the migration, proliferation, differentiation, and survival of monocytes and macrophages by binding to CSF-1R and blocking its activation by its two known ligands, CSF-1 and IL-34. It offers a novel therapeutic option for treatment of these pts
Methods: SNDX-6352-0503 is a Phase 1/2 dose-escalation and dose-expansion study evaluating safety, tolerability, pharmacokinetics (PK)/pharmacodynamics (PD), and efficacy of axa in pts >6 years of age with active symptomatic cGVHD despite ≥2 prior lines of therapy. The Phase 1 endpoints were safety, tolerability, PK and PD with the primary objective of defining optimal biologic dose; the primary endpoint of the Phase 2 study is overall response rate (CR+PR) by 6 months. Pts were dosed in 28-day cycles. The data cutoff was 22 July 2020.
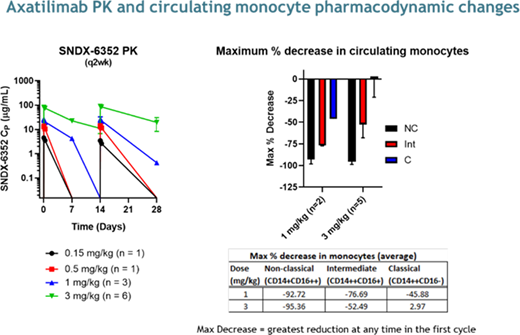
Results: Twelve pts have been enrolled in the Phase 1 study. Median age at enrollment was 58y (range, 29-73y), 8 pts were male. Pts had failed a median of 5 prior lines of treatment (range 4-9). Doses included 0.15 mg/kg (n=1), 0.5mg/kg (n=1), 1mg/kg (n=3), 3 mg/kg (n=6) every 2 weeks (q2w), and 3mg/kg q4w (n=1). Of these, 5 pts (42%) are still receiving axa. The median number of cycles for all pts is 5 (range 1-12). Of the 3 pts whose starting dose was 3 mg/kg q2w and remain on study, 2 dose reduced; one to 2 mg/kg q4w and one to 1 mg/kg q2w. Seven pts (58%) discontinued due to: adverse events (3 mg/kg q2w, n=2); death due to traumatic fall (1 mg/kg q2w, n=1); investigator decision (0.5 mg/kg q2w, n=1); progressive cGVHD (1 and 0.15 mg/kg q2w, n=1 each); and non-compliance (3 mg/kg q2w, n=1).
Two of 6 pts (17%) at a dose of 3 mg/kg q2w reported a treatment emergent adverse event that was considered a dose limiting toxicity (DLT): 1 with CTCAE Grade 4 creatine kinase increase with symptoms of myositis after dose 1, and the 2nd with an elevation in amylase/lipase that delayed the 3rd dose for >2 weeks. The latter pt restarted therapy at 1 mg/kg q2w and remains on treatment after 5 cycles.
Four pts (1 at 0.15 mg/kg and 3 at 3 mg/kg q2w, 33%) had a related treatment emergent adverse event that was ≥Grade 3: increase in aspartate aminotransferase (n=2); increase in creatine phosphokinase (n=2); and increase in gamma-glutamyl transferase (n=2). Such biochemical elevations may be a consequence of CSF-1R blockade on Kupffer cells leading to an inhibition in the clearance of these enzymes, consistent with the mechanism of action of axa and when asymptomatic have not been associated with clinical manifestations of hepatitis, pancreatitis, or rhabdomyolysis. Periorbital edema was observed in 2 pts (≤Grade 2); no additional CSF-1Ri class-effect associated TEAEs were observed
Clinical responses as defined by the 2014 NIH cGVHD Consensus Criteria have been observed in 7 pts (58%) across all dose levels; median time to response was 12 weeks. Organ-specific responses have been observed in esophagus (n=1/1), eyes (n=3/10), joints/fascia (n=5/9), mouth (n=1/7), and skin (n=3/8). Prior therapies received by the responders included ibrutinib (6 pts), ruxolitinib (5 pts), and KD025 (3 pts); 3 of the responding pts had received all of these. Six pts (50%) reported at least a 7-point improvement in the Lee Symptom Score. Preliminary PK profiles and pharmacodynamic endpoints, including circulating CD14+CD16+ nonclassical and CD14++CD16+ intermediate monocyte kinetics, are consistent with those observed in healthy volunteers and pts with solid tumors see figure below.
Conclusions: These data demonstrate that axatilimab is clinically active with acceptable safety profile and responses observed in heavily pretreated pts with active cGVHD. Enrollment continues in the Phase 1 study at 3 mg/kg q4w and Phase 2 study at a dose of 1 mg/kg q2w.
Arora:Syndax: Research Funding; Fate Therapeutics: Consultancy; Pharmacyclics: Research Funding; Kadmon: Research Funding. Jagasia:Janssen: Research Funding; Mallinckrodt: Research Funding; Ocugen: Other. Meyers:Syndax Pharmaceuticals, Inc: Current Employment. Quaranto:Syndax Pharmaceuticals, Inc: Current Employment. Schmitt:Syndax Pharmaceuticals, Inc: Current Employment. Sankoh:Syndax Pharmaceuticals, Inc: Current Employment. Abu Zaid:Syndax: Research Funding. Hill:Roche: Research Funding; Generon: Consultancy; CSL: Research Funding; Implicit Bioscience: Research Funding; Pharmacyclics: Research Funding; Compass Pharmaceuticals: Research Funding. Weisdorf:Incyte: Research Funding; FATE Therapeutics: Consultancy. Blazar:BlueRock Therapeuetic: Consultancy; Magenta Therapeutics: Consultancy; BlueRock Therapeutics: Research Funding; Childrens' Cancer Research Fund: Research Funding; KidsFirst Fund: Research Funding; Tmunity: Other: Co-founder; Fate Therapeutics Inc.: Research Funding. Ordentlich:Syndax Pharmaceuticals, Inc: Current Employment. Lee:Takeda: Research Funding; AstraZeneca: Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Research Funding; Novartis: Research Funding; Kadmon: Research Funding; Incyte: Consultancy, Research Funding; Syndax: Research Funding.
The drug (Axatilimab) is a humanized antibody against CSF-1R. The study is a phase I trial testing this drug in patients with chronic graft versus host disease
Author notes
Asterisk with author names denotes non-ASH members.